Abstract
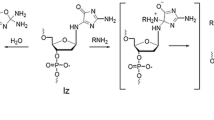
Methylglyoxal and glyoxal are generated from the oxidation of carbohydrates and lipids, and like d-glucose have been shown to nonenzymatically react with proteins to form advanced glycation end products (AGEs). AGEs can occur both in vitro and in vivo, and these compounds have been shown to exacerbate many of the long-term complications of diabetes. Earlier studies in our laboratory reported d-glucose, d-galactose, and d/l-glyceraldehyde formed AGEs with nucleosides. The objective of this study was to focus on purines and pyrimidines and to analyze these DNA nucleoside derived AGE adducts with glyoxal or methylglyoxal using a combination of analytical techniques. Studies using UV and fluorescence spectroscopy along with mass spectrometry provided for a thorough analysis of the nucleoside AGEs and demonstrated that methylglyoxal and glyoxal reacted with 2′-deoxyguanosine via the classic Amadori pathway, and did not react appreciably with 2′-deoxyadenosine, 2′-deoxythymidine, and 2′-deoxycytidine. Additional findings revealed that methylglyoxal was more reactive than glyoxal.









Similar content being viewed by others
References
Neglia CL, Cohen HJ, Gaber AR, Ellis PP, Thorpe SR, Baynes JW (1983) J Biol Chem 258:14279–14283
Dutta U, Cohenford MA, Dain JA (2005) Anal Biochem 345:171–180
Wautier JL, Guillausseau PJ (2001) Diabetes Metab 27:535–542
Sensi, M (1995) Diabetes Res Clin Pract 28:9–17
Li YY, Dutta U, Cohenford MA, Dain JA (2007) Bioorg Chem 35:417–429
Ahmed N, Howell S, Smith K, Szwergold B (2003) Biochim Biophys Acta 1639:121–132
Thornalley PJ (1996) Gen Pharmacol 27:565–573
Fukunaga M, Miyata S, Higo S, Hamada Y, Ueyama S, Kasuga M (2005) Ann N Y Acad Sci 1043:151–157
Karachalias M, Babaei-Jadidi R, Ahmed N, Thornalley PJ (2003) Biochem Soc Trans 31:1423–1425
Beisswenger P, Howell S, Nelson R (2003) Biochem Soc Trans 31:1358–1363
Li YY, Cohenford MA, Dutta U, Dain JA (2007) FASEB J 21:499.2
Cohenford MA, Urbanowski JC, Shepard DC, Dain JA (1983) Immunol Commun 12:189–200
Chen SP, Huang T, Sun SG (2005) J Chromatogr A 1089:142–147
Khuhawar MY, Kandhro AJ, Khand FD (2006) Anal Lett 39:2205–2215
De Sa PFG, Treubig JM, Brown PR, Dain JA (2001) Food Chem 72:379–384
Miyata T, Kurokawa K, Van Ipersele de Strihon C (2000) J Am Soc Nephrol 11:1744–1752
Biemel KM, Reihl O, Conrad J, Lederer MO (2001) J Biol Chem 26:23405–23412
Vlassara H, Striker LJ, Teichberg S, Fuh H, Li YM, Steffes M (1994) Proc Natl Acad Sci USA 91:11704–11708
Daniels BS, Hauser EB (1992) Diabetes 41:1415–1421
Brownlee M (1994) Diabetes 43:836–841
Colaco CA, Harrington CR (1994) Neuroreport 5:859–861
Li H, Nakamura S, Miyazaki S, Morita T, Suzuki M, Pischetsrieder M, Niwa T (2006) Kidney Int 69:388–392
Dutta U, Cohenford MA, Dain JA (2006) Anal Bioanal Chem 386:1633–1640
Acknowledgements
This research was made possible by the use of Research and Bioinformatics Core Facilities supported jointly by NCRR/NIH grant no. P20 RR016457 and the Network institutions and by funds that were gifted by Monica Hatfield to Marshall University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Figures
(PDF 271 kb)
Rights and permissions
About this article
Cite this article
Li, Y., Cohenford, M.A., Dutta, U. et al. The structural modification of DNA nucleosides by nonenzymatic glycation: an in vitro study based on the reactions of glyoxal and methylglyoxal with 2′-deoxyguanosine. Anal Bioanal Chem 390, 679–688 (2008). https://doi.org/10.1007/s00216-007-1682-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-007-1682-4