Abstract
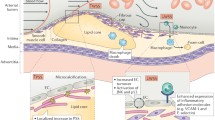
In this study, we hypothesized that spatial relationships exist between the local mechanical environment and inflammatory marker expression in atherosclerotic plaques, and that these relationships are plaque-progression dependent. Histologic cross-sections were collected at regular intervals along the length of diseased human coronary arteries and classified as early, intermediate, advanced, or mature based on their morphological features. For each cross-section, the spatial distribution of stress was determined using a 2D heterogeneous finite element model, and the corresponding distribution of selected inflammatory markers (macrophages, matrix metalloproteinase-1 [MMP-1], and nuclear factor-kappa B [NF-κB]) were determined immunohistochemically. We found a monotonic spatial relationship between mechanical stress and activated NF-κB that was consistent in all stages of plaque progression. We also identified progression-dependent relationships between stress and both macrophage presence and MMP-1 expression. These findings add to our understanding of the role of mechanical stress in stimulating the inflammatory response, and help explain how mechanical factors may regulate complex biological changes in remodeling.
Similar content being viewed by others
References
Aplin AE, Howe A, Alahari SK et al (1998) Signal transduction and signal modulation by cell adhesion receptors: The role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol Rev 2(50): 197–261
Arroyo LH, Lee RT (1999) Mechanisms of plaque rupture: mechanical and biologic interactions. Cardiovasc Res 2(41): 369–375
American Heart Association (2006) Heart disease and stroke statistics—2006 update. Dallas, Texas
Beattie D, Xu C, Vito R et al (1998) Mechanical analysis of heterogeneous, atherosclerotic human aorta. J Biomech Eng 5(120): 602–607
Cheng GC, Loree HM, Kamm RD et al (1993) Distribution of circumferential stress in ruptured and stable atherosclerotic lesions. A structural analysis with histopathological correlation. Circulation 4(87): 1179–1187
Davies MJ (1998) Reactive oxygen species, metalloproteinases, and plaque stability. Circulation 24(97): 2382–2383
Finet G, Ohayon J, Rioufol G (2004) Biomechanical interaction between cap thickness, lipid core composition and blood pressure in vulnerable coronary plaque: Impact on stability or instability. Coron Artery Dis 1(15): 13–20
Galis ZS, Sukhova GK, Lark MW et al (1994) Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest 6(94): 2493–2503
Gao X, Iwai M, Inaba S et al (2007) Attenuation of monocyte chemoattractant protein-1 expression via inhibition of nuclear factor-kappab activity in inflammatory vascular injury. Am J Hypertens 11(20): 1170–1175
Grote K, Flach I, Luchtefeld M et al (2003) Mechanical stretch enhances mrna expression and proenzyme release of matrix metalloproteinase-2 (mmp-2) via nad(p)h oxidase-derived reactive oxygen species. Circ Res 11(92): e80–e86
Halloran BG, Grange JJ, So BJ et al (1997) Macrophage products inhibit human aortic smooth muscle cell proliferation and alter 1 alpha (i) procollagen expression. Ann Vasc Surg 1(11): 80–84
Hallow KM (2007) Relationships between mechanical stress and markers of inflammation in diseased human coronary arteries. School of Mechanical Engineering, Georgia Institute of Technology. Available via SMARTech. http://smartech.gatech.edu/handle/1853/16211
Hanley JA, Negassa A, Edwardes MD et al (2003) Statistical analysis of correlated data using generalized estimating equations: An orientation. Am J Epidemiol 4(157): 364–375
Harrison DG, Widder J, Grumbach I et al (2006) Endothelial mechanotransduction, nitric oxide and vascular inflammation. J Intern Med 4(259): 351–363
Hishikawa K, Oemar BS, Yang Z et al (1997) Pulsatile stretch stimulates superoxide production and activates nuclear factor-kappa b in human coronary smooth muscle. Circ Res 5(81): 797–803
Humphrey JD (2002) Cardiovascular solid mechanics: cells, tissues, and organs. Springer, New York
Ingber DE (2002) Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res 10(91): 877–887
James TW, Wagner R, White LA et al (1993) Induction of collagenase and stromelysin gene expression by mechanical injury in a vascular smooth muscle-derived cell line. J Cell Physiol 2(157): 426–437
Jiang MJ, Yu YJ, Chen YL et al (1999) Cyclic strain stimulates monocyte chemotactic protein-1 mrna expression in smooth muscle cells. J Cell Biochem 2(76): 303–310
Keeny S, Richardson PD (1987) Stress analysis of atherosclerotic arteries. IEEE Eng Med Biol 9: 1484–1485
Kumar A, Takada Y, Boriek AM et al (2004) Nuclear factor-kappab: its role in health and disease. J Mol Med 7(82): 434–448
Lee RT, Libby P (1997) The unstable atheroma. Arterioscler Thromb Vasc Biol 10(17): 1859–1867
Lee RT, Loree HM, Cheng GC et al (1993) Computational structural analysis based on intravascular ultrasound imaging before in vitro angioplasty: Prediction of plaque fracture locations. J Am Coll Cardiol 3(21): 777–782
Lee RT, Schoen FJ, Loree HM et al (1996) Circumferential stress and matrix metalloproteinase 1 in human coronary atherosclerosis. Implications for plaque rupture. Arterioscler Thromb Vasc Biol 8(16): 1070–1073
Lehoux S, Castier Y, Tedgui A (2006) Molecular mechanisms of the vascular responses to haemodynamic forces. J Intern Med 4(259): 381–392
Lendon CL, Davies MJ, Born GV et al (1991) Atherosclerotic plaque caps are locally weakened when macrophages density is increased. Atherosclerosis 1(87): 87–90
Liu J (1999) Biomechanical basis of vascular tissue engineering. Crit Rev Biomed Eng 1-2(27): 75–148
Loree HM, Kamm RD, Stringfellow RG et al (1992) Effects of fibrous cap thickness on peak circumferential stress in model atherosclerotic vessels. Circ Res 4(71): 850–888
Lowder ML, Li S, Carnell PH et al (2007) Correction of distortionof histologic sections of arteries. J Biomech 2(40): 445–450
Moreno PR, Purushothaman KR, Fuster V et al (2004) Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: Implications for plaque vulnerability. Circulation 14(110): 2032–2038
Nikkari ST, O’Brien KD, Ferguson M et al (1995) Interstitial collagenase (mmp-1) expression in human carotid atherosclerosis. Circulation 6(92): 1393–1398
Ohayon J, Teppaz P, Finet G et al (2001) In-vivo prediction of human coronary plaque rupture location using intravascular ultrasound and the finite element method. Coron Artery Dis 8(12): 655–663
Ohki R, Yamamoto K, Mano H et al (2002) Identification of mechanically induced genes in human monocytic cells by DNA microarrays. J Hypertens 4(20): 685–691
Pasterkamp G, Schoneveld AH, van der Wal AC et al (1999) Inflammation of the atherosclerotic cap and shoulder of the plaque is a common and locally observed feature in unruptured plaques of femoral and coronary arteries. Arterioscler Thromb Vasc Biol 1(19): 54–58
Rekhter MD (1999) Collagen synthesis in atherosclerosis: Too much and not enough. Cardiovasc Res 2(41): 376–384
Robbesyn F, Salvayre R, Negre-Salvayre A (2004) Dual role of oxidized ldl on the NF-κB signaling pathway. Free Radic Res 6(38): 541–551
Sakamoto H, Aikawa M, Hill CC et al (2001) Biomechanical strain induces class a scavenger receptor expression in human monocyte/macrophages and thp-1 cells: A potential mechanism of increased atherosclerosis in hypertension. Circulation 1(104): 109–114
Stary HC (2000) Natural history and histological classification of atherosclerotic lesions: An update. Arterioscler Thromb Vasc Biol 5(20): 1177–1178
Stary HC, Chandler AB, Dinsmore RE et al (1995) A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. Circulation 92: 1355–1374
Taber LA, Humphrey JD (2001) Stress-modulated growth, residual stress, and vascular heterogeneity. J Biomech Eng 6(123): 528–535
Takahashi K, Takeya M, Sakashita N (2002) Multifunctional roles of macrophages in the development and progression of atherosclerosis in humans and experimental animals. Med Electron Microsc 4(35): 179–203
van der Wal AC, Becker AE, van der Loos CM et al (1994) Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 1(89): 36–44
Vito R, Whang MC, Giddens DP et al (1990) Stress analysis of the diseased arterial cross-section. ASME Adv Bioeng Proc 19: 273–276
Yamamoto K, Ikeda U, Shimada K (2003) Role of mechanical stress in monocytes/macrophages: implications for atherosclerosis. Curr Vasc Pharmacol 3(1): 315–319
Yang JH, Sakamoto H, Xu EC et al (2000) Biomechanical regulation of human monocyte/macrophage molecular function. Am J Pathol 5(156): 1797–1804
Zampetaki A, Zhang Z, Hu Y et al (2005) Biomechanical stress induces il-6 expression in smooth muscle cells via ras/rac1-p38 mapk-NF-κB signaling pathways. Am J Physiol Heart Circ Physiol 6(288): H2946–H2954
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hallow, K.M., Taylor, W.R., Rachev, A. et al. Markers of inflammation collocate with increased wall stress in human coronary arterial plaque. Biomech Model Mechanobiol 8, 473–486 (2009). https://doi.org/10.1007/s10237-009-0151-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-009-0151-8